Use Lewis dot structures to show the ionic bonding in the following pairs of elements. Show the transfer of electrons using arrows. Write the correct chemical formula for the ionic compound that forms.
1) barium oxide (Ba and O)
2) sodium oxide (Na and O)
3) calcium chloride (Ca and Cl)
4) sodium nitride (Na and N)
5) aluminum oxide (Al and O)
6) magnesium phosphide (Mg and P)
Answers
Answer:
See explanation
Explanation:
A Lewis structure is a representation of atoms of elements using dots. These dots show the number of outermost electrons in the atom. These outermost electrons are involved during ionic bonding.
We can see from the image attached that in ionic bonding, electrons are transferred from the metal to the non metal to form the ionic compound. Arrows have been used to show the transfer of electrons from metals to non metals.
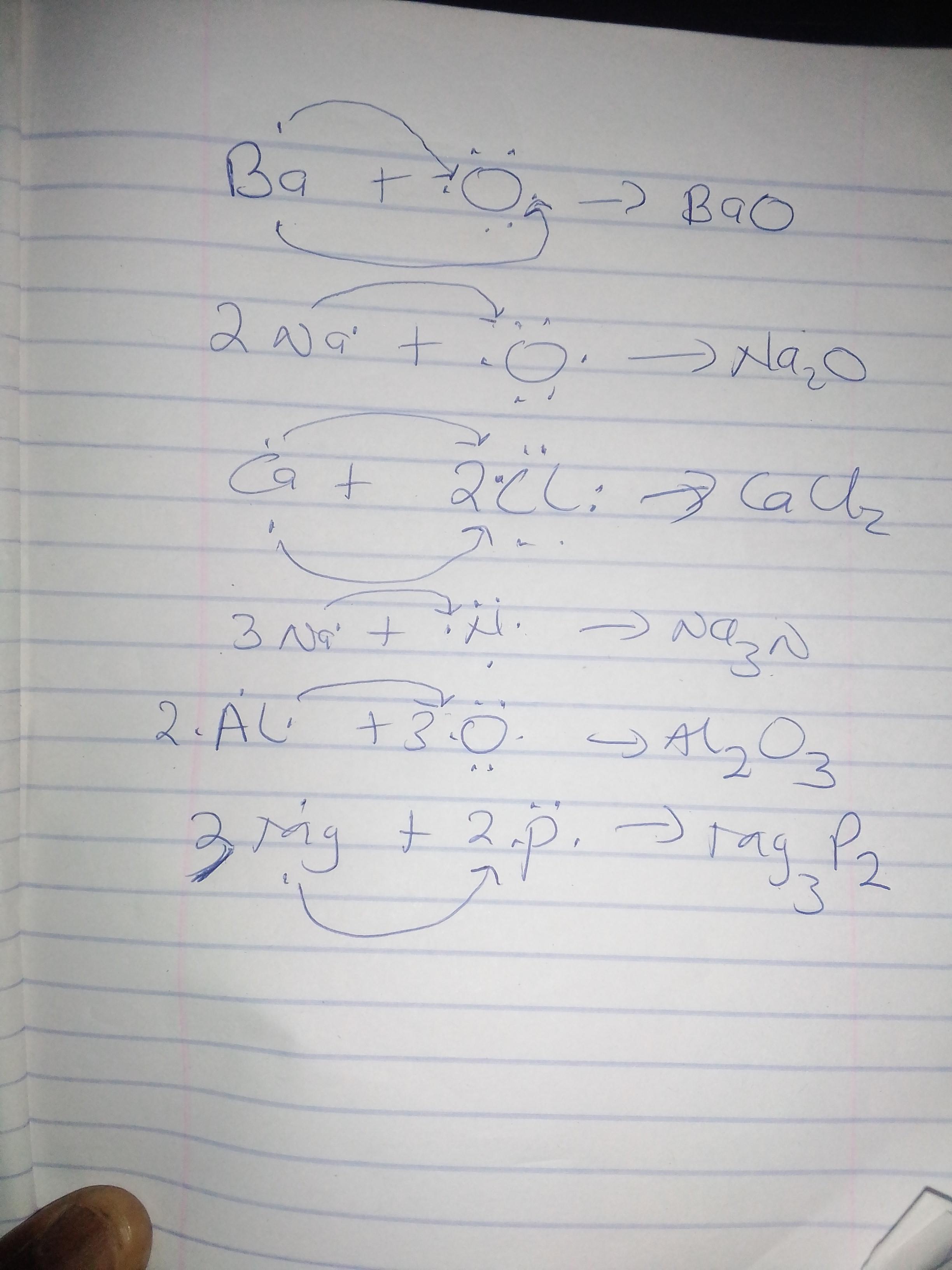
Related Questions
What is the gravitational force on a 35.0kg object standing on the earth's surface ?
Answers
Answer:
Explanation:
F=mg
F=35*9.81
F=343.35N
Limiting Reactant
12.0 grams of sodium reacts with 5.00 grams of chlorine. What mass of sodium
chloride could be produced?
Nas) +
Cl2(g) →
NaCls)
(1)
(2)
Identify the limiting reactant.
Determine the amount of sodium chloride produced.
Answers
Answer:
(1) Cl₂ is the limiting reactant.
(2) 8.18 g
Explanation:
2Na(s) + Cl₂(g) → 2NaCl(s)First we convert the given masses of reactants into moles, using their respective molar masses:
Na ⇒ 12.0 g ÷ 23 g/mol = 0.522 mol NaCl₂ ⇒ 5.00 g ÷ 70.9 g/mol = 0.070 mol Cl₂0.070 moles of Cl₂ would react completely with (2 * 0.070) 0.14 moles of Na. There are more Na moles than that, so Na is the reactant in excess while Cl₂ is the limiting reactant.
Then we calculate how many moles of NaCl are formed, using the limiting reactant:
0.070 mol Cl₂ * [tex]\frac{2molNaCl}{1molCl_2}[/tex] = 0.14 mol NaClFinally we convert NaCl moles into grams:
0.14 mol NaCl * 58.44 g/mol = 8.18 gAssume the copper was not thoroughly dried. How will the following calculations be affected?
The calculated mass of copper will be too low or too high?
The calculated moles of copper will be too low or too high?
The calculated mass of chlorine will be too low or too high?
The calculated moles of chlorine will be too low or too high?
Answers
How many moles of KBr are present in 1000 ml of a 0.02 M KBr solution?
1. .00002
2. 20
3. 50000
4. .02
5. 50
Answers
Answer:
4. .02
Explanation:
Hello there!
In this case, since the molarity of a solution is defined as the moles of the solute divided by the volume of the solution, we can see we are given the molarity and volume and are asked to compute moles; thus, we can solve as shown below:
[tex]M=\frac{n}{V}\\\\n=M*V[/tex]
Whereas the volume must be in liters (1 L in this case); in such a way we can plug in the volume and molarity to obtain:
[tex]n=0.02mol/L*1L\\\\n=0.02mol[/tex]
Therefore, the answer is 4. .02 .
Best regards!
What is thrust force
Answers
Answer:
The force that moves a plane forward through the air. Thrust is created by a propeller or a jet engine. An aircraft in straight and level flight is acted upon by four forces: lift, gravity, thrust, and drag. The opposing forces balance each other; lift equals gravity and thrust equals drag.
What atom has the largest atomic radius barium calcium magnesium radium
Answers
Answer:
Radium
Explanation:
It has the highest atomic number so it would have more shells, making it larger than the others.
Instruction
Active
TRY IT Writing Measurements in Scientific Notation
Choose the scientific notation that best represents the standard notation given.
6,840,000,000 m
68.4 x 108 m
6.84 x 108 m
6.84 x 10-9 m
6.84 x 109 m
0.000000000143 g
-1.43 x 1010 g
1.43 x 10-10 g
14.3 x 1010 g
14.3 x 10-10 g
COMPLETE
DONE
Answers
Answer:
D & B
Explanation:
just did it
Which level is made up of organisms that break down dead organisms?
Answers
Answer:
Explanation:
decomposers
What is the correct shape and polarity of a water molecule
Answers
Answer:
D
Explanation:
because both H's should be positive and O is supposed to be negative.
how many moles of nitrogen gas are produced in the reaction if we started with 6 moles of ammonium nitrate
Answers
The molar ratio between Nitrogen and ammonia is
1
:
3
, therefore, to produce 18 moles of ammonia, we will need
What information does the percent composition of an atom in a molecule
give?
Answers
Answer:
percent composition tells that by mass
hi, if your looking for extra points (50+) and brainiest here is ur chance, answer this question correctly plz
Answers
Answer:
Answer choice 'C'
Explanation:
As the ball slows after kicking generates maximum Kinetic Energy, the ball slows due to its Kinetic Energy being transferred to surroundings because of atmospheric and surface friction until it stops in a state of static equilibrium.
sorry if im wrong, but im sure its either one.
The human body can get energy by metabolizing proteins, carbohydrates or fatty acids, depending on the circumstances. Roughly speaking, the energy it gets comes mostly from allowing all the carbon atoms in the food molecules to become oxidized to carbon dioxide CO2 by reaction with oxygen from the atmosphere. Hence the energy content of food is roughly proportional to the carbon content.
Let's consider alanine, C3H7NO2, one of the amino acids from which proteins are made, and glucose C6H12O6, one of the simplest carbohydrates. Using the idea above about energy content, calculate the ratio of the energy the body gets metabolizing each gram of alanine to the energy the body gets metabolizing each gram of glucose.
Answers
Answer:
the ratio of the energy the body gets metabolizing each gram of alanine to the energy the body gets metabolizing each gram of glucose is 1.0111
Explanation:
Given the data in the question;
To determine the ratio of the energy the body gets metabolizing each gram of alanine to the energy the body gets metabolizing each gram of glucose, first we get the molar masses of both alanine and glucose
we know that;
Molar mass of alanine ( C₃H₇NO₂ ) = 89.09 g/mol
Molar mass of glucose ( C₆H₁₂O₆ ) = 180.16 g/mol
now, { metabolizing each gram }
moles of alanine = mass taken / molar mass
= 1g / 89.09 g/mol = 1/89.09 moles
moles of glucose = mass taken / molar mass
= 1g / 180.16 g/mol = 1/180.16 moles
In each molecule of alanine, we have 3 atoms of carbon.
Also, in each molecules of glucose, we have 6 atoms of carbon
so,
number of moles of Carbons in alanine = 3 × 1/89.09 moles = 0.03367
number of moles of Carbons in glucose = 6 × 1/180.16 moles = 0.0333
so ratio of energy will be the ratio of carbon atoms, which is;
⇒ 0.03367 / 0.0333 = 1.0111
Therefore, the ratio of the energy the body gets metabolizing each gram of alanine to the energy the body gets metabolizing each gram of glucose is 1.0111
Sam and Jane have been investigating the amount of copper sulphate that can be dissolved in water at different temperatures .They added copper sulphate till it could not be dissolved any further and also measured the mass of copper. The results are below. What conclusions can you make from results?
Answers
Answer:
This question is so confusing, I'm sorry
Name the process that happens when a liquid turns into a gas.
Answers
When a liquid is insoluble in another liquid, the liquids are said to be?
Answers
Answer:
immiscible
Explanation:
molar mass of Mg(CO3)
Answers
Answer:
[tex]\boxed {\boxed {\sf 84.313 \ g/mol}}[/tex]
Explanation:
The molar mass is the mass per mole of an element or substance. The values for atomic mass on the Periodic Table are used as the molar mass. Look up the molar masses for the elements in the formula.
Mg: 24.305 g/molC: 12.011 g/molO: 15.999 g/molAnalyze the formula. Magnesium and carbon have no subscript, so there is only one atom of each. Oxygen has a subscript of 3, so there are 3 atoms and the mass has to be multiplied by 3.
MgCO₃= 24.305 + 12.011+ 3(15.999) MgCO₃= 24.305+12.011+47.997=84.313 g/molThe molar mass of magnesium carbonate is 84.313 grams per mole.
What are the dependent and independent variables? (Picture^^)
Answers
Answer:
X axis shows the independent and Y axis shows the dependent.
Explanation:
As the independent variable is always plotted on the X-axis of the graph the Y-axis shows the dependent variable that is affected by the X variables Time is an independent variable and is always shown along the X-axis. Such as the image showed tells about the data of N2O biofuel emissions from 2009-10. Thus this will be plotted along the X-axis and Y-axis will show the amount of nitrogen fertilizer applied.2 CH3OH + 3 02 2 CO2 + 4H2O
What is the mass of oxygen (O2) that is required to produce 579 g of carbon dioxide (CO2)?
. Your answer should have three significant figures.
Answers
Answer:
632 g
Explanation:
2CH₃OH + 3O₂ → 2CO₂ + 4H₂OFirst we convert 579 g of CO₂ into moles, using its molar mass:
579 g CO₂ ÷ 44 g/mol = 13.16 mol CO₂Then we convert CO₂ moles into O₂ moles, using the stoichiometric coefficients:
13.16 mol CO₂ * [tex]\frac{3molO_2}{2molCO_2}[/tex] = 19.74 mol O₂Finally we convert O₂ moles into grams, using its molar mass:
19.74 mol O₂ * 32 g/mol = 632 gWhich of the following explains why a longer bond is also a weaker bond? Help plz
Answers
Answer:
Longer bonds have lower attractive force
pond water with microscopic organisms inside mixture or pure substance
Answers
Answer:
it's mixture
Explanation:
since pond is composed of not only microbes and also more aqatic animals so I think pond become mixture but not pure substance am I right or not...
How many formula units make up 10.2 g of magnesium chloride (MgCl2)?
Answers
Answer:
6.46×10²² formula units
Explanation:
From the question given above, the following data were obtained:
Mass of MgCl₂ = 10.2 g
Number of formula units =?
From Avogadro's hypothesis,
1 mole of MgCl₂ = 6.02×10²³ formula units
But,
1 mole of MgCl₂ = 24 + (35.5×2) = 24 + 71 = 95 g
Thus, we can say:
95 g of MgCl₂ = 6.02×10²³ formula units
Finally, we shall determine the formula units in 10.2 g MgCl₂. This can be obtained as follow:
95 g of MgCl₂ = 6.02×10²³ formula units
Therefore,
10.2 g of MgCl₂ = (10.2 × 6.02×10²³) / 95
10.2 g of MgCl₂ = 6.46×10²² formula units
Thus, 10.2 g of MgCl₂ contains 6.46×10²² formula units
Lets be honest here no one would help me with these 3-4 questions but anyways
Answers
Answer:
Continental drift was a theory that explained how continents shift position on Earth's surface. Set forth in 1912 by Alfred Wegener, a geophysicist and meteorologist, continental drift also explained why look-alike animal and plant fossils, and similar rock formations, are found on different continents.
Explanation:
nun
Answer:
Continental drift or tectonic plates is a theory that explains the formation of the current surface structure of the Earth.
Continental drift is also understandable if you take pictures of each continents map and try to join them with another, for example, try to fit the map of western south India with Oman's southern region you will see they exactly fit each other, Science explains this as that the land had split and drifted to form what we see now, and this is known as continental drift or tectonic plates. This form was brought up by Alfred Wegener in 1912.
This theory is also the reasoning for the rising of mountains like the Himalayan mountains, which are having a rise in their height over time, the Himalayan mountains are believed to be belonging to a boundary of two plates that move towards each other and this is believed as the rise of India.
A compound contains 0.5 mol Na, 0.5 mol N, and 10 mol H. The empirical formula of the
compound is -
Answers
Answer:
NaNH₂₀
Explanation:
0.5 mol Na, 0.5 mol N, and 10 mol H
To obtain the empirical formulae, we find the mole ratio between the elements and this is done by dividing all through by the smallest mol (0.5)
Na = 0.5 / 0.5 = 1
N = 0.5 / 0.5 = 1
H = 10 / 0.5 = 20
The mole ratio is used to write the empirical formulae. It is given as;
NaNH₂₀
Who tryna do these 3 assignments on wizer for me
Answers
what is a 7 letter word that starts with f: Ancient plants and animals formed into rocks.
Answers
Answer:
Fossils?
Explanation:
A falling ball looses its shape and bounces off the ground because it has...Immersive Reader
Kinetic energy
Potential energy
Elastic energy
Thermal energy
Answers
Please someone help me with this!!
Answers
1.00 x 10^6 atoms of gold is equivalent to how many grams?
Answers
Answer:
3.27 x 10⁻¹⁶ grams
Explanation:
moles Au = 1.00 x 10⁻⁶ Atoms / 6.02 x 10²³Atoms / mole = 1.66 x 10⁻¹⁸ mole Au
grams Au = 1.66 x 10⁻¹⁸ mole Au x 196.97 grams Au/mole Au
= 3.27 x 10⁻¹⁶ grams Au
Sulfur dioxide and oxygen react to form sulfur trioxide during one of the key steps in sulfuric acid synthesis. An industrial chemist studying this reaction fills a flask with of sulfur dioxide gas and of oxygen gas, and when the mixture has come to equilibrium measures the partial pressure of sulfur trioxide gas to be . Calculate the pressure equilibrium constant for the reaction of sulfur dioxide and oxygen at the final temperature of the mixture. Round your answer to significant digits.
Answers
Answer:
The answer is "[tex]\bold{4.97 \times 10^{-2}}[/tex]"
Explanation:
Please find the complete question in the attached file.
Equation:
[tex]2SO_2+O_2 \leftrightharpoons 2SO_3[/tex]
at [tex]t=0 3.3 \ \ \ \ \ \ \ \ \ \ 0.79[/tex]
at equilibrium [tex]3.3-p \ \ \ \ \ \ \ \ \ \ 0.79 - \frac{P}{2} \ \ \ \ \ \ \ \ \ \ \ \ P[/tex]
[tex]p= 0.47 \ \ atm\\\\SO_2=3.3-0.47 = 2.83 \ \ atm\\\\O_2= 0.74 -\frac{0.47}{2}=0.74-0.235=0.555 \ atm\\\\K_P=\frac{[PSO_3]^2}{[PSO_2]^2[PO_2]}\\\\[/tex]
[tex]=\frac{0.47^2}{2.83^2\times 0.555}\\\\=4.97 \times 10^{-2}[/tex]