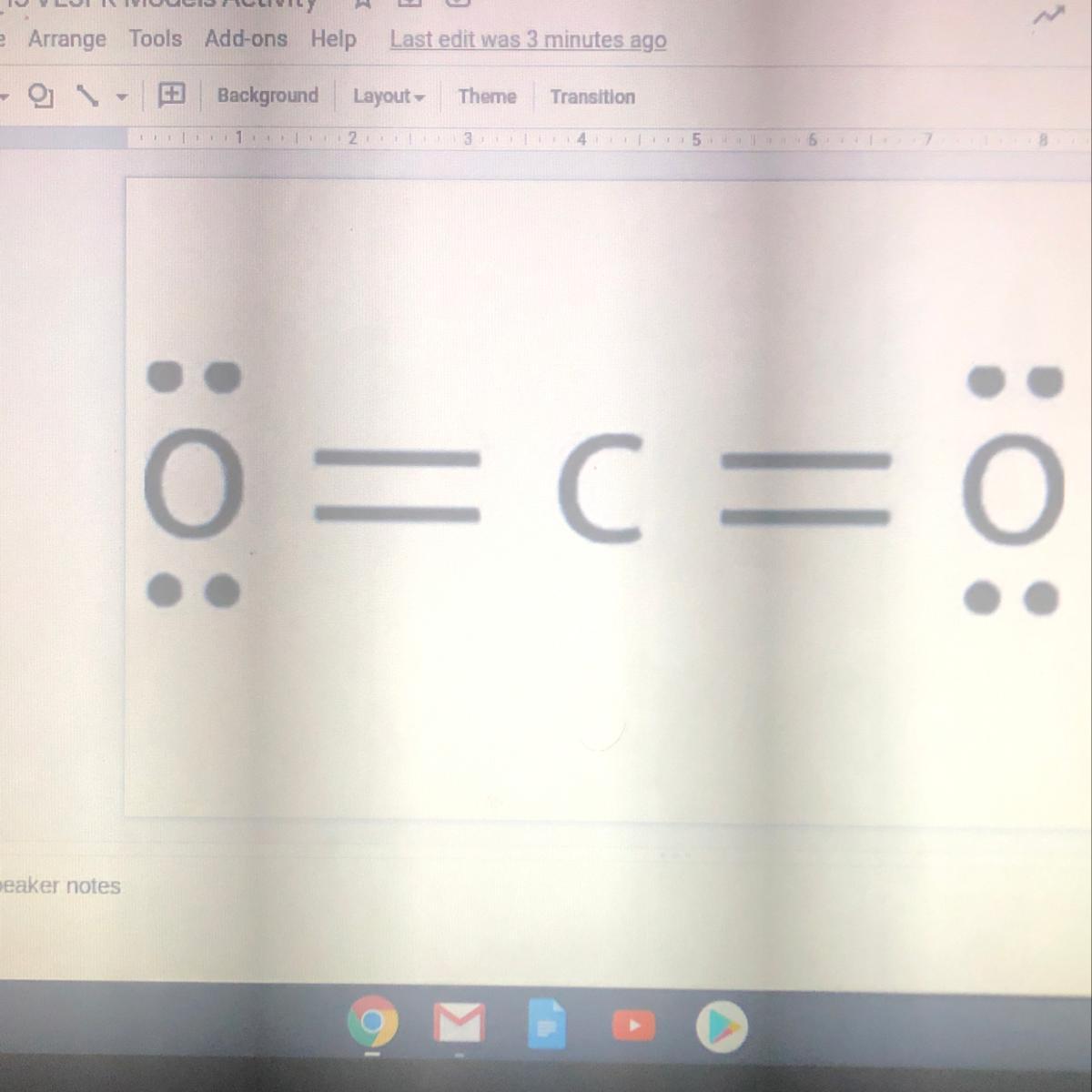
Answers
Answer:
carbon dioxide CO[tex]{2}[/tex]
Explanation:
Double Polar Covalent bonds with CO2
Explanation:
Keep in mind that CO2 is a nonpolar molecule
Related Questions
Which waves have the highest frequencies?
A. microwaves
B.UV
C. waves with the lowest energy
D. waves with the highest energy
Answers
Answer:
D. Waves with the highest energy
Explanation:
The more energy the higher the frequency. More specifically there will be more energy per photon.
Microwaves are on the low end of the electromagnetic spectrum while UV is higher but there are more waves with more energy such as gamma rays.
5. Aidan is on a carnping trip with his family. As the temperature decreases
Aidan suggests building a campfire. Which of the following explains why
heat moves from the campfire to the people sitting around it? *
Answers
Answer:
Because heat flows from warmer objects to cooler objects
Explanation:
5. The diagram above shows what happened when an irregular solid was immersed in water. This irregular solid has the same mass as a gold block, which is 2 cm wide by 2 cm high by 5 cm long. Using the method described in the passage, compare the density of the unknown with the density of gold.
A. Equal to gold
B. Less than gold
C. Greater than gold
D. Cannot be determined
Answers
The density of the unknown will be greater than the density of gold.
An overview will be given since the diagram isn't given. The formula to calculate the density of an irregular shape will be the mass divided by the volume.
The density is the mass per volume. From the information given, since they've the same mass, then it means that the volume of the gold will be more than the volume of the unknown. Therefore, the density of the unknown will be greater than gold.
Read related link on:
https://brainly.com/question/18370124
Un compuesto formado por carbono, hidrógeno y oxígeno tiene una masa de 4,6 g. Se hace reaccionar con 9,6 g de oxígeno dando 8,8 g de CO2 y 5,4 g de agua. Si cogemos 9,2 g de un compuesto en un volumen 5,80l en P= 780 mmHg a una temperatura de 90ºC. Calcula la fórmula empírica y molecular.
Answers
Answer:
La fórmula empírica y molecular es: C₂H₆O.
Explanation:
Para calcular la formula empírica y molecular del compuesto debemos primero plantear la reacción:
[tex] C_{x}H_{y}O_{z} + O_{2} \rightarrow CO_{2} + H_{2}O [/tex]
Necesitamos encontrar "x", "y" y "z". Para ello, tenemos que recordar que la masa de carbono e hidrógeno producida está relacionada con la cantidad de C y H inicial (del compuesto):
Para el H:
CHO → H₂O
y 5,4g
[tex] \frac{2*1 g}{18 g} = \frac{y}{5,4 g} \rightarrow y = 0,6 g [/tex]
Para C:
CHO → CO₂
x 8,8g
[tex] \frac{12 g}{44 g} = \frac{x}{8,8 g} \rightarrow x = 2,4 g [/tex]
Para el O:
[tex] z = 4,6 g - 2,4 g - 0,6 g = 1,6 g [/tex]
Ahora mediante el calculo de los moles del C, H y O podemos encontrar la fórmula empírica:
Para el H:
[tex] n_{y} = \frac{m}{Pm} = \frac{0,6 g}{1 g/mol} = 0,6 moles [/tex]
Para el C:
[tex] n_{x} = \frac{m}{Pm} = \frac{2,4 g}{12 g/mol} = 0,2 moles [/tex]
Para el O:
[tex] n_{z} = \frac{m}{Pm} = \frac{1,6 g}{16 g/mol} = 0,1 moles [/tex]
[tex] C_{\frac{n_{x}}{n_{z}}}H_{\frac{n_{y}}{n_{z}}}O_{\frac{n_{z}}{n_{z}}} = C_{\frac{0,2}{0,1}}H_{\frac{0,6}{0,1}}O_{\frac{0,1}{0,1}} = C_{2}H_{6}O_{1} [/tex]
Entonces, la fórmula empírica del commpuesto formado es C₂H₆O.
Ahora para determinar la fórmula molecular podemos usar la siguiente relación:
[tex] \frac{Pm}{Pm_{e}} = n [/tex]
[tex] F_{m} = n*F_{e} [/tex]
[tex] F_{m} = \frac{Pm}{Pm_{e}}*F_{e} [/tex]
En donde Fm (fórmula molecular) y Fe (fórmula empírica) están relacionadas por n.
El valor de Pm lo obtenemos de la ecuación del gas ideal:
[tex]PV = nRT = \frac{m}{Pm}RT[/tex]
[tex] Pm = \frac{mRT}{PV} = \frac{9,2 g*0,082 L*atm/(K*mol)*(90 + 273 K)}{1.02 atm*5,80 L} = 46,3 g/mol [/tex]
[tex] F_{m} = \frac{46,3 g/mol}{(2*12 + 6*1 + 16)g/mol}*C_{2}H_{6}O_{1} = 1.00*C_{2}H_{6}O_{1} = C_{2}H_{6}O_{1} [/tex]
Por lo tanto, la fórmula molecular es la misma que la fórmula empírica, a saber C₂H₆O.
Espero que te sea de utilidad!
which molecule or ion has a linear shape?
a. BeCl2
b. PH3
c. ClO4-
d. PCl5
Answers
Answer:
bec12 is it.one or i it is under 180
25 cm of liquid 'A' and 20 cm of liquid
'B' are mixed at 25°C and the volume of
solution was measured to be 44.8 cm3
then correct reaction is
(A) A Hmix = 0, solution shows
ideal
Answers
Answer:
The correct option is;
(B) [tex]\Delta H_{mix} < 0[/tex], solution shows negative deviation
Explanation:
The given parameters are;
The available volume of liquid A = 25 cm³
The available volume of liquid B = 20 cm³
The volume of the solution (mixture) = 44.8 cm³
Therefore, we have;
[tex]\Delta _{mix} V < 0[/tex]
Which is one of the prerequisite for the formation of negative deviation
When a non-ideal solution shows negative deviation according to Raoult's Law, we have;
[tex]\Delta _{mix} H < 0[/tex], we have more heat released due to new molecular interactions.
Not all protists use flagella or cilia to move. Give an example of another way protists move and identify the type of protist that uses that mode of movement.
Answers
Answer: One of the most striking features of many protist species is the presence of some type of locomotory organelle, easily visible under a light microscope. A few forms can move by gliding or floating, although the vast majority move by means of “whips” or small “hairs” known as flagella or cilia, respectively. (Those organelles give their names to informal groups—flagellates and ciliates—of protists.) A lesser number of protists employ pseudopodia. Those same organelles may be used in feeding as well.
Explanation:
Brownian motion is
A. random movement of particles suspended in a fluid
B. movement of particles from an area of high concentration to low concentration
C. movement of particles from an area of low concentration to high concentration
D. random movement of smaller particles
Answers
Answer: A.
Explanation:
Brownian motion is the random motion of a particle as a result of collisions with surrounding gaseous molecules. Diffusiophoresis is the movement of a group of particles induced by a concentration gradient. This movement always flows from areas of high concentration to areas of low concentration.
Example: The movement of pollen grains on still water. Motion of dust motes in a room (though largely influenced by air currents).
3. The nucleus:
a. controls all the cell activities
b. controls only part of the cell
c. does not have any activity
Answers
Answer:
a. controls all the cell activities.Thank you ☺️
Use the rules of significant figures to report the answer to this math question: (5.003 x 10^4) - (6.059 x 10^3) + (9.23 x 10^2) =
Answers
Answer:
44894
Explanation:
Material
Specific Heat
(J/gºC)
Use this chart showing the different specific heat of
various materials to answer the questions.
Which material has the highest specific heat? waterv
Which material has the lowest specific heat?
Which material has the ability to absorb twice as much
heat as aluminum when placed in the same
environment of mass and temperature?
aluminum
0.90
iron
0.44
lead
0.16
sand
0.83
steel
0.49
wood
1.80
water
4.18
) Intro
Done
6 of 9
Answers
Answer:
1. C
2. A
3. B
Explanation:
I took the Assignment
The material with highest specific heat is water.
The material with lowest specific heat is lead.
The material that has the ability to absorb twice as much heat as aluminium is wood.
What is specific heat? Specific heat is the quantity of heat that are required to raise the temperature of one gram of a substance by one Celsius degree. The units of specific heat are usually calories or joules per gram per Celsius degree.To learn more about specific heat here
https://brainly.com/question/18812365
#SPJ2
what is the median number of 69,64,63,66?
Answers
First put them in order from least to greatest. Then remove outer numbers. You are then left with 64 and 66. The number in the middle of 64 and 66 is 65.
Calculate the net force
175 N
1125 N
Answers
Answer:
_____________&_________Net force = sum of all forces with directionsso.....NET FORCE = 125+(-75) = 50 N in the direction of yellow t-shirt man------------------------------------How does the atomic radii trend explain the electronegativity trend?
Answers
Answer: electronegativity decreases.
Explanation: This is because atomic number increases down a group, and thus there is an increased distance between the valence electrons and nucleus, or a greater atomic radius.
please help this is due in 30 minutes!!
Answers
Answer:
Hello. The answer is A (10)
Explanation:
If you memorize the periodic table it will be very easy to answer.
But if you don't know،
The solution is to count the electrons number
In an atom usually electrons number is equal with protons number
In this shape there are 10 electrons
So there are 10 protons in nucleus.
which statement correctly describes protons and neutrons?
a. they have the same mass and the same electrical charge
b. they have the same mass but different electrical charge
c. they have the different masses but the same electrical charge
d. they have different masses and different electrical charges
Answers
Answer:
B
Explanation:
Which term describes erosion?
A.creates small particles
B. Hardens rock fragments
C. Transports solid materials
D. dissolves rock components
Answers
Stan gets frustrated while working on his math homework, and he tears his paper into several pieces. What kind of change has his
paper gone through?
Answers
Answer:
Physical Change
Explanation:
What is a physical change? A physical change is a change to the physical—as opposed to chemical—properties of a substance. They are usually reversible. The physical properties of a substance include such characteristics as shape (volume and size), color, texture, flexibility, density, and mass.
What is the volume of kristas rock
Answers
Answer : The volume of kristas rock is 30 mL.
Explanation :
From the given image we conclude that:
Initial volume of liquid = 150 mL
Final volume of liquid = 180 mL
Now we have to determine the volume of kristas rock.
Volume of kristas rock = Final volume of liquid - Initial volume of liquid
Volume of kristas rock = 180 mL - 150 mL
Volume of kristas rock = 30 mL
Therefore, the volume of kristas rock is 30 mL.
lonic bonding
10. If two atoms that differ in electronegativity combine by chemical reaction and
share electrons, the bond that joins them will be
(1 Point)
ionic bond
polar covalent
nonpolar covalent
a hydrogen bond
Answers
Answer:
polar covalent
Explanation:
If they do not equally share the electron then it is polar and covalent bonds share electrons
what are the units for the volume of a solid volume of a liquid
Answers
Answer:Volume is the quantity of three-dimensional space enclosed by a closed surface, for example, the space that a substance (solid, liquid, gas, or plasma) or shape occupies or contains.
Explanation:
How does Newton’s laws explain motion
Answers
Explanation:
Newton's second law is a quantitative description of the changes that a force can produce on the motion of a body. It states that the time rate of change of the momentum of a body is equal in both magnitude and direction to the force imposed on it.
What happens to an egg that is not fertilized?
OIt is reabsorbed by the body
O It travels back up the fallopian tube.
OIt exits during childbirth.
O It is released during menstruation
Answers
Answer:
D. It is released during menstruation
Explanation:
The egg that is not fertilized is released during the process of menstruation.
What is menstruation?
Menstruation (also known as a period, among other colloquial terms) is the regular discharge of blood and mucosal tissue from the inner lining of the uterus through the vagina. The menstrual cycle is characterized by the rise and fall of hormones. Menstruation is triggered by falling progesterone levels and is a sign that pregnancy has not occurred.
The first period, a point in time known as menarche, usually begins between the ages of 12 and 15.Menstruation starting as young as 8 years would still be considered normal.The average age of the first period is generally later in the developing world, and earlier in the developed world. The typical length of time between the first day of one period and the first day of the next is 21 to 45 days in young women. In adults, the range is between 21 and 31 days with the average being 28 days.
Learn more about menstruation,here:
https://brainly.com/question/9864045
#SPJ7
The protons and neutrons are in the nucleus of the atom, while the electrons are on the outside. How would you calculate the number of neutrons in an atom? Subtract the atomic mass and the atomic number. Add the atomic mass and the atomic number. Subtract the number of protons from the atomic number. Add the number of protons to the atomic number.
Answers
what is a well-tested, explanation that unifies a broad range of observations and hypotheses
Answers
Dilute hydrochloric acid reacts with sodium
2HCl(aq) + Na2CO3(aq) + 2NaCl(aq) + H2O(l) + CO2(g)
A Explain why effervescence is seen during the reaction.
Answers
Answer:
Because the reaction releases CO2 gas
Explanation:
therefore creating an effervecence within the solution as the carbonate dissociates
How are electrically neutral atoms different than they form
Answers
An atom is said to be electrically neutral if it has an equal amount of protons and electrons. On the other hand, an atom is electrically charged if its protons and electrons are not distributed evenly.
What are neutral atoms?Neutral atoms are defined as when an atom has an equal amount of protons and electrons, it also has an equal number of electric charges, both positive and negative. As a result, every element in the periodic table has a neutral atomic structure.
Normal atoms have an equal amount of positive and negative particles and a neutral charge. Accordingly, an atom with a neutral charge is one in which the atomic number is matched by the number of electrons.
Thus, an atom is said to be electrically neutral if it has an equal amount of protons and electrons. On the other hand, an atom is electrically charged if its protons and electrons are not distributed evenly.
To learn more about neutral atoms, refer to the link below:
https://brainly.com/question/5308494
#SPJ2
Quick
Choch
Place each description in the correct category.
represented by symbols
Elements
Compounds
made of one type of atom
represented by formulas
cannot be broken down
can be chemically broken down
made of two or more types of atoms
Answers
Answer:
a i believe
Explanation:
a
what turns colour in an acid and base
Answers
Answer:
Chemists use a solution called Universal Indicator to identify acids and bases. ... The Universal Indicator Color Guide shows that Universal Indicator turns red when it is added to a strong acid, it turns purple when it is added to a strong base, and it turns a yellowish-green when it is added to a neutral solution.
Explanation:
Answer:
Phenolphthalein
Explanation:
What is the mass in grams of each of the following?
a. 2.50 mol of sulfur, S
b. 1.80 mol of calcium, Ca
c. 0.50 mol of carbon, C
d. 3.20 mol of copper, Cu
Answers
a) 80
b) 36
c)6
d)64
All in grams
the formula ; n= m
M
was used .
where n is moles
m is mass of a substance
M is molar mass
The mass of the substance is the product of the moles and the molar mass. The mass of sulfur = 80 gm, calcium = 72 gm, carbon = 6 gm, and copper = 201.6 gm.
What is mass?Mass of any substance is the amount of the constituent particle it holds together and can be measured in grams. The mass has been calculated from the moles of the substance and its molar mass.
The mass of sulfur is calculated as,
Mass = moles × molar mass
= 2.50 mol × 32 g/mol
= 80 gms
The mass of calcium is calculated as,
Mass = moles × molar mass
= 1.80 mol × 40 g/mol
= 72 gms
The mass of carbon is calculated as,
Mass = moles × molar mass
= 0.50 mol × 12 g/mol
= 6 gms
The mass of copper is calculated as,
Mass = moles × molar mass
= 3.20 mol × 63 g/mol
= 201.6 gms
Learn more about mass and moles here:
https://brainly.com/question/20970268
#SPJ5