Answers
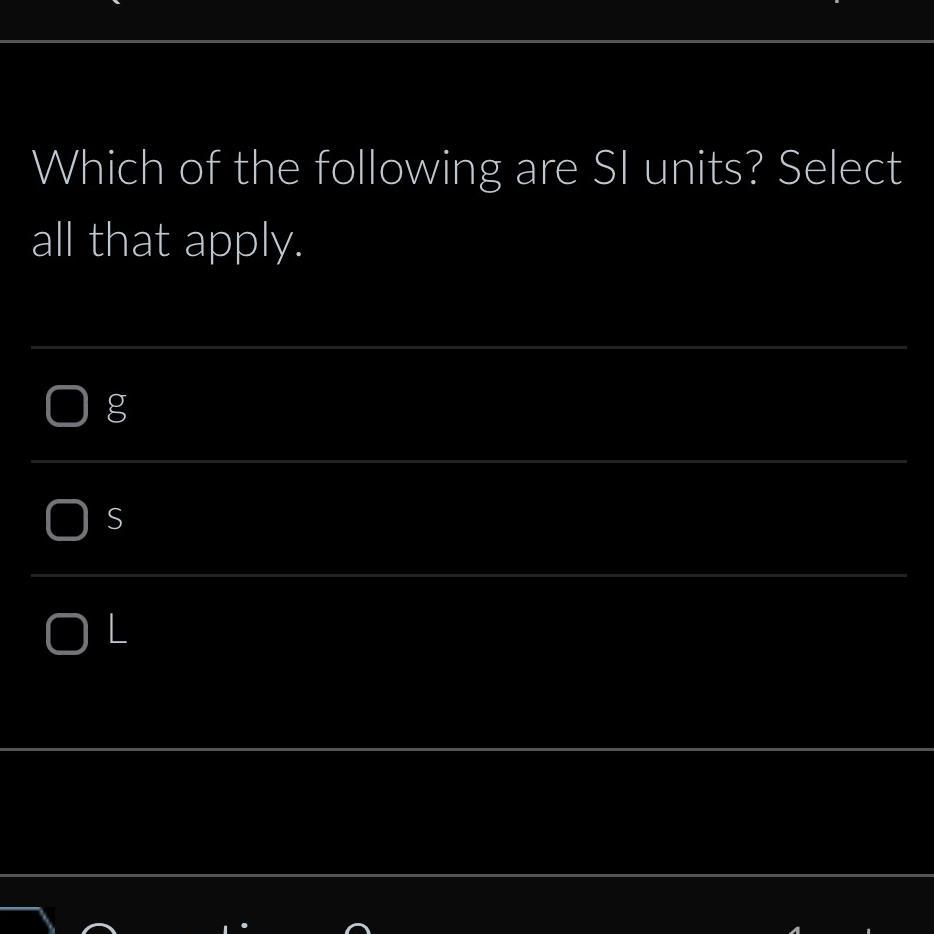
Seconds (s)
Liters (L)
Explanation:SI units relate to the International System of Units. These units are the base, metric units that are most commonly accepted for different measurements.
Common SI Units
The most common SI units are as follows:
Length - meter (m)Time - second (s)Mass - kilogram (kg)Amount of substance - mole (mole)Electric current - ampere (A)Temperature - kelvin (K)Volume - liter (L)*Note that gram (g) is not an SI unit
Each type of measurement will only have 1 SI unit. This is the unit that will be expected for most answers. Sometimes this means converting an answer into a different unit so it is more widely accepted.
Why SI Units are Important
SI units are important for the same reason that the metric system is used. It gives a standardized list of measurements that scientists across the world can use. Also, it makes it easier to compare scientific findings and studies when all of the measurements are given in the same units.
There are times when it is unrealistic to use SI units. For example, when discussing space, using meters will not be realistic due to the size of space. Also, when measuring the mass of small elements, kilograms are too large. But otherwise, SI units should be used.
Related Questions
The presence of the radioactive gas radon (Rn) in well water obtained from aquifers that lie in rock deposits presents a possible health hazard in parts of the United States.
a)Assuming that the solubility of radon in water with 1 atm pressure of the gas over the water at 30 degrees c is 7.27x10^-3 M, what is the Henry's law constant for radon in water at this temperature?
b)A sample consisting of various gases contains 3.7×10-6 mole fraction of radon. This gas at a total pressure of 31atm is shaken with water at 30 degrees c. Calculate the molar concentration of radon in the water.
Answers
The Henry's law constant for radon in water at 30°C is 2.24 x 10^-2 M/atm. The molar concentration of radon in the water when shaken with a gas containing 3.7 x 10^-6 mole fraction of radon at a total pressure of 31 atm is 2.63 x 10^-7 M.
a) To calculate the Henry's law constant (K_H) for radon in water at 30°C, use the formula:
K_H = C_gas / P_gas
where C_gas is the molar concentration of radon in water (7.27 x 10^-3 M) and P_gas is the pressure of radon gas over the water (1 atm). Plugging in the values:
K_H = (7.27 x 10^-3 M) / (1 atm) = 7.27 x 10^-3 M/atm
b) To calculate the molar concentration of radon in the water, first find the partial pressure of radon in the gas mixture:
P_Rn = mole fraction of radon x total pressure = (3.7 x 10^-6) x (31 atm) = 1.147 x 10^-4 atm
Now, use the Henry's law constant (K_H) to find the molar concentration of radon in water:
C_Rn = K_H x P_Rn = (7.27 x 10^-3 M/atm) x (1.147 x 10^-4 atm) = 2.63 x 10^-7 M
Know more about Henry Law Constant here:
https://brainly.com/question/30636760
#SPJ11
Four students measured The acceleration of gravity the excepted value for the location is 9. 78 mi. /s squared which students measurement has the largest percent error
Answers
To determine which student's measurement has the largest percent error in measuring the acceleration of gravity, we need to calculate the percent error for each student's measurement and compare them to the expected value of 9.78 m/s^2. The percent error is calculated by subtracting the expected value from the measured value, dividing by the expected value, and multiplying by 100.
The student with the largest percent error will have the measurement that deviates the most from the expected value.
Explanation:
To calculate the percent error for each student's measurement, we can use the formula:
Percent Error = |(Measured Value - Expected Value) / Expected Value| * 100
Let's assume the measured values for the four students are A, B, C, and D.
The percent error for each student can be calculated as follows:
Percent Error(A) = |(A - 9.78) / 9.78| * 100
Percent Error(B) = |(B - 9.78) / 9.78| * 100
Percent Error(C) = |(C - 9.78) / 9.78| * 100
Percent Error(D) = |(D - 9.78) / 9.78| * 100
By comparing the calculated percent errors for each student, we can determine which measurement has the largest percent error. The student with the largest percent error will have the measurement that deviates the most from the expected value of 9.78 m/s^2.
To learn more about Gravity - brainly.com/question/31321801
#SPJ11
1. If we used 8. 7 g sunflower oil and recover 7. 8 g FAMEs, what is the weight % yield for this
reaction? Report your answer to the nearest whole number
TABLE 1 Fatty acid composition of some oils (w/w%). The symbol "Cxx. Y" denotes the
number of carbon atoms in the carboxylic acid, xx, and the number of cis double bonds in the
hydrocarbon chain, y.
Oil
Myristic
Acid
C14:0
8
Palmitic
Acid
C16:0
Oleic
Acid
C18:1
22
Stearic
Acid
C18:0
0
3
3
Linoleic
Acid
C18:2
5
54
Linolenic
Acid
C18:3
0
17
Cod liver
Cottonseed
Olive
1
19
1
22
13
0
71
10
1
Safflower
0
7
2
13
78
0
Sesame
0
9
4
41
45
0
Sunflower 0
7
5
19
68
1
Note: The solid fats contain significant amounts of C10-C14 fatty acids and tend to have
unsaturated saturated fatty acid ratios of < 1 (w/w).
Answers
The weight % yield of the reaction, to determine the percentage of the desired product (FAMEs) obtained from the starting material (sunflower oil).
Given:
Mass of sunflower oil used = 8.7 g
Mass of FAMEs recovered = 7.8 g
Weight % yield is calculated using the formula:
Weight % yield = (Mass of desired product / Mass of starting material) × 100
Substituting the given values:
Weight % yield = (7.8 g / 8.7 g) × 100
Weight % yield = 89%
Therefore, the weight % yield for this reaction is approximately 89% when 8.7 g of sunflower oil is used, and 7.8 g of FAMEs are recovered.
In its most basic form, it typically refers to a production process or its result. The term "producers" is used by economists to describe derived organisations. These companies think about marketing products to customers. For instance, a textile company might produce and market garments for customers.
Learn more about reaction here
https://brainly.com/question/30464598
#SPJ11
in chapter 13 you learned that the bonding forces in ionic solids such as nacl are very strong yet many ionic solids dissolve readily in water explain
Answers
The strong bonding forces in ionic solids are due to the electrostatic attraction between positively and negatively charged ions. When an ionic solid is introduced to water, the polar water molecules surround the ions and weaken the ionic bonds through a process called hydration.
This process involves the formation of new electrostatic interactions between water molecules and the ions, where the partially negative oxygen atom of water is attracted to the positively charged ion and the partially positive hydrogen atoms are attracted to the negatively charged ion.
As more and more water molecules surround the ions, the ions become separated from each other and eventually dissolve in the water. The extent to which an ionic solid dissolves in water depends on the strength of the hydration energy relative to the lattice energy of the solid.
To know more about the strong bonding forces refer here :
https://brainly.com/question/14719148#
#SPJ11
Complete question :
In chapter 13, you learned that the bonding forces in ionic solids such as NaCl are very strong, yet many ionic solids dissolve readily in water. Explain.
Why did we count the drops of stearic acid solution in 1 ml?
Answers
Counting drops of stearic acid solution in 1 ml is crucial for maintaining accuracy, consistency, and reliability in scientific experiments. This practice allows researchers to control conditions, draw conclusions, and ensure that their results can be compared and reproduced in future studies.
It's essential to count the drops of stearic acid solution in 1 ml to ensure accurate measurement and consistency in a scientific experiment. Stearic acid is a saturated fatty acid commonly used in various applications, such as chemistry, biology, and materials science. By counting the drops, researchers can determine the concentration of stearic acid in a given volume and control the experimental conditions.
Accurate measurements are crucial in experiments to produce reliable and reproducible results. Counting the drops helps maintain precision and allows for the correct interpretation of data. When comparing outcomes or replicating experiments, a consistent methodology, including accurate measurements of solutions, is necessary for obtaining valid conclusions.
Moreover, understanding the concentration of stearic acid in 1 ml is essential for calculations and analysis related to the specific experiment. For example, researchers may need to determine the percentage of stearic acid in a compound or its solubility in various solvents. Precise measurement of the number of drops in 1 ml helps in these calculations, ensuring that the conclusions drawn are based on accurate data.
Know more about fatty acid here:
https://brainly.com/question/30712004
#SPJ11
Provide the correct iupac name for cu(c₂h₃o₂)₂
Answers
The correct IUPAC name for Cu(C₂H₃O₂)₂ is Copper(II) Acetate.
Your answer: The correct IUPAC name for Cu(C₂H₃O₂)₂ is copper(II) acetate. This name is derived by following these steps:
1. Identify the cation (metal) in the compound, which is copper (Cu).
2. Identify the anion (non-metal) in the compound, which is acetate (C₂H₃O₂).
3. Determine the oxidation state of the copper. Since there are two acetate ions, each with a charge of -1, the copper must have a +2 charge.
4. Combine the names of the cation and anion, specifying the oxidation state of the cation in parentheses as a Roman numeral: copper(II) acetate.
To know more about Copper visit:
https://brainly.com/question/13677872
#SPJ11
Calculate ΔG for this reaction at 25 ∘C under the following conditions:
PCH3OH= 0.845 atm
PCO= 0.115 atm
PH2= 0.160 atm
CH3OH(g)⇌CO(g)+2H2(g)
Answers
The ΔG for the reaction at 25 °C from the given conditions is -11.43 kJ/mol.
What is the standard Gibbs free energy change at 25 °C for the given reaction?In this reaction, CH3OH(g) is converting to CO(g) and 2H2(g). To calculate the ΔG at 25 °C, we need to use the equation ΔG = ΔG° + RT ln(Q), where ΔG° is the standard Gibbs free energy change, R is the gas constant (8.314 J/(mol·K)), T is the temperature in Kelvin (298 K for 25 °C), and Q is the reaction quotient.
To calculate Q, we need to determine the partial pressures of the gases involved. Given that P(CH3OH) = 0.845 atm, P(CO) = 0.115 atm, and P(H2) = 0.160 atm, we can substitute these values into the equation.
Using the ideal gas law, we can convert the partial pressures to concentrations: [CH3OH] = P(CH3OH)/RT, [CO] = P(CO)/RT, and [H2] = P(H2)/RT.
Next, we substitute the concentrations into the reaction quotient expression: Q = ([CO]·[H2]^2) / [CH3OH].
Finally, plugging in the values into the ΔG = ΔG° + RT ln(Q) equation and solving for ΔG, we find that the standard Gibbs free energy change for the given reaction at 25 °C is -11.43 kJ/mol.
Learn more about reaction
brainly.com/question/14444620
#SPJ11
the phosphates that make up the phosphodiester bonds in dna have pka 2. when the ph of solution is dropped to 2.5, what is the charge of c. elegans dna, which is 97,000-kilo-base-pairs (kbp) long?
Answers
At pH 2.5, the phosphates in DNA are fully protonated and positively charged due to the low pH. The pKa of the phosphates is 2, so at pH 2.5, most of the phosphates will be protonated. As a result, DNA at this pH will have a positive charge.
The length of the DNA molecule is given as 97,000 kilobase pairs (kbp), which is a measure of the number of nucleotide pairs in the DNA. To calculate the charge of the DNA.
We need to know the number of phosphates in the molecule, which is equal to twice the number of nucleotide pairs. Therefore, the number of phosphates in the DNA is 194,000.
Since each phosphate group carries a charge of -1 at neutral pH, the total charge on the DNA at pH 2.5 can be calculated by subtracting the number of protons from the total number of phosphates.
At pH 2.5, the number of protons is equal to 10^(2.5-2) times the number of phosphates, or 194,000 * 0.1 = 19,400. Thus, the net charge on the DNA at pH 2.5 is 194,000 - 19,400 = 174,600 elementary charges, or 1.746 x 10⁵ C.
To know more about DNA, refer here:
https://brainly.com/question/21992450#
#SPJ11
what is the temperature at which Deuterium-tritium fusion occurs and cite this value in terms of Kelvin. How strong must the magnets in these experiments be to contain the resultant plasma?
Answers
Deuterium-tritium fusion occurs at a temperature of approximately 100 million Kelvin (100 MK) or 15 keV. This is much higher than the temperature at the core of the sun, which is around 15 million Kelvin.
To contain the resultant plasma, strong magnetic fields are used to confine the hot, ionized gas. The strength of these magnetic fields is typically measured in units of tesla (T).
The required magnetic field strength depends on the specific experimental setup, but typical values range from several tesla to tens of tesla.
The stronger the magnetic field, the better the confinement of the plasma. However, the design of the magnets and the materials used to construct them must also take into account other factors such as thermal and mechanical stresses, radiation damage, and cost.
To learn more about the fusion, follow the link:
https://brainly.com/question/12701636
#SPJ1
arrange the following elements in order of increasing electronegativity: carbon, fluorine, oxygen, nitrogen
Answers
Based on these values, we can arrange the elements in order of increasing electronegativity as follows: Carbon (C) < Nitrogen (N) < Oxygen (O) < Fluorine (F)
Electronegativity is the measure of an atom's ability to attract electrons towards itself in a chemical bond. The electronegativity of an atom increases as we move towards the upper right-hand corner of the periodic table. Therefore, in order of increasing electronegativity, the given elements can be arranged as follows:
Carbon (C) < Nitrogen (N) < Oxygen (O) < Fluorine (F)
Carbon has the lowest electronegativity of all the given elements. Nitrogen has a slightly higher electronegativity than carbon, followed by oxygen, and finally, fluorine has the highest electronegativity of all the given elements.
The reason for fluorine's high electronegativity is that it has a small atomic size and a large nuclear charge. This means that the attraction between the positively charged nucleus and the negatively charged electrons is very strong. Oxygen also has a relatively high electronegativity because it has a similar atomic structure to fluorine.
In summary, when arranging the given elements in order of increasing electronegativity, the order is Carbon (C) < Nitrogen (N) < Oxygen (O) < Fluorine (F).
To know more about electronegativity, refer to the link below:
https://brainly.com/question/21669668#
#SPJ11
Calculate the hydroxide ion concentration, hydronium ion concentration and the pH of a 0.10 M CH3NH2 solution. (Kb = 4.2 x 104). Write a chemical equation showing the relevant equilibrium.
Answers
The pH of the solution is 13.16. And the equation is CH3NH2 + H2O ⇌ CH3NH3+ + OH-
The relevant equilibrium for the reaction of CH3NH2 (methylamine) with water is:
CH3NH2 + H2O ⇌ CH3NH3+ + OH-
The equilibrium constant expression is:
Kb = [CH3NH3+][OH-]/[CH3NH2]
We can use this expression to find the concentration of hydroxide ions, [OH-]:
Kb = [CH3NH3+][OH-]/[CH3NH2]
4.2 x 10^4 = x^2 / 0.10
x = 0.145 M
Therefore, the concentration of hydroxide ions in the solution is 0.145 M.
To find the concentration of hydronium ions, [H3O+], we can use the equation:
Kw = [H3O+][OH-]
1.0 x 10^-14 = [H3O+][0.145]
[H3O+] = 6.90 x 10^-14 M
Therefore, the concentration of hydronium ions in the solution is 6.90 x 10^-14 M.
To find the pH of the solution, we can use the equation:
pH = -log[H3O+]
pH = -log(6.90 x 10^-14)
pH = 13.16
Therefore, the pH of the solution is 13.16.
Chemical equation for the reaction:
CH3NH2 + H2O ⇌ CH3NH3+ + OH-
For more such questions on pH , Visit:
https://brainly.com/question/172153
#SPJ11
In a 0.10 M CH3NH2 solution, the hydroxide ion concentration is 0.00205 M, the hydronium ion concentration is 4.88 x 10⁻¹² M, and the pH is approximately 11.31.
What are the hydroxide ion concentration, hydronium ion concentration, and pH of a 0.10 M CH₃NH₂ solution?The chemical equation showing the relevant equilibrium is as follows:
CH₃NH₂ + H₂O ⇌ CH₃NH₃+ + OH⁻
Data given:
Initial concentration of CH₃NH₂ = 0.10 M
Kb = 4.2 x 10⁻⁴
Let x be the concentration of hydroxide ions (OH⁻) formed and also the concentration of CH₃NH₃⁺ formed.
The initial concentration of CH₃NH₂ is 0.10 M, and since it is a weak base, we assume it does not significantly dissociate, and the change in concentration is negligible.
At equilibrium,
[CH₃NH₂ ] = (0.10 - x) M,
[CH₃NH₃⁺] = x M, and
[OH⁻] = x M.
Solving for x;
4.2 x 10⁻⁴ = x * x / (0.10 - x)
Since Kb is small compared to 0.10, we assume that (0.10 - x) ≈ 0.10.
4.2 x 10⁻⁴ = x² / 0.10
x² = 4.2 x 10⁻⁴ * 0.10
x ≈ 0.00205 M
The hydronium ion concentration (H₃O⁺) is calculated as follows:
Kw = [H₃O⁺][OH⁻]
1.0 x 10⁻¹⁴ = [H₃O⁺]
[H₃O⁺] ≈ 4.88 x 10^(-12) M
Therefore;
pH = -log[H₃O⁺]
pH = -log(4.88 x 10⁻¹²)
pH ≈ 11.31
Learn more about hydroxide ion concentration and pH at: https://brainly.com/question/11755508
#SPJ4
an electron in the n=1 bohr orbit has the kinetic energy k1 . in terms of k1 , what is the kinetic energy of an electron in the n=2 bohr orbit?
Answers
The kinetic energy of an electron in the n=2 Bohr orbit can be expressed in terms of k1 as k2 = k1/4. This is because the kinetic energy of an electron in a Bohr orbit is proportional to 1/n^2, where n is the principal quantum number.
To determine the kinetic energy of an electron in the n=2 Bohr orbit in terms of k1 which is the kinetic energy of an electron in the n=1 Bohr orbit, we can do the following :
Step 1: Understand the relationship between kinetic energy and the Bohr orbits.
In the Bohr model, the kinetic energy of an electron is inversely proportional to its orbit number (n).
Step 2: Use the proportionality formula for kinetic energy in Bohr orbits.
The formula to calculate kinetic energy in relation to the orbit number is:
Kn = K1 / n^2
Step 3: Calculate the kinetic energy for the n=2 Bohr orbit.
Using the formula from Step 2, plug in n=2:
K2 = K1 / (2^2)
Step 4: Simplify the equation.
K2 = K1 / 4
The kinetic energy of an electron in the n=2 Bohr orbit is K1/4, where K1 is the kinetic energy of an electron in the n=1 Bohr orbit.
Learn more about kinetic energy : https://brainly.com/question/8101588
#SPJ11
What is the definition of beam spreading in science?
Answers
Answer:
Beam spreading is the result of small-angle scattering, resulting in increased beam divergence and reduced spatial power density at the receiver.
Explanation:
FILL IN THE BLANK. As gas reactants are compressed into a smaller volume, increasing the reactants partial pressures, the number of collisions _______ and the rate of reaction ___________.
Answers
Answer:
Both no. of collisions and rate of reaction INCREASES
Consider an electrochemical cell with a zinc electrode immersed in 1.0 M electrode immersed in 0.10 M Zn^2+ and a nickel electrode immersed in 0.10 M Ni^2+
Zn^2+ + 2e^- ---> Zn E degree = -0.76 V
Ni^2+ + 2e^- ---> Ni E degree = -0.23 V
33. [Algorithmic] Calculate E for this cell.
a). 0.53 V b). 0.50 V c). 0.56 V d). 0.47 V e). 0.59 V
34. Calculate the concentration of Ni^2+ if the cell is allowed to run to equilibrium at 25 degree C.
a). 1.10 M
b). 0.20 M
c). 0.10 M
d). 0M
e). none of these
Answers
33. The E for the cell is 0.53 V,
34. The concentration of the Ni²⁺ is 0 M.
33. The reactions are :
Ni²⁺ + 2e⁻ ----> Ni E° = -0.23 V
Zn --> Zn²⁺ + 2e⁻ E° = 0.76 V
The cell potential = - 0.23 V + 0.76 V
The cell potential = 0.53 V
34. The change in the concentration for the ions of the solution will affect the cell potential :
Ecell = E°cell - (0.0592 V / n) log Q
As the reaction proceeds to the equilibrium, the Ni²⁺ decreases and the Zn²⁺ decreases.
0.53 = (0.0592 / 2) log (0.1 + x / 0.1 - x)
x = 0.1 M
[Ni²⁺] = 0.1 - 0.1 = 0 M.
To learn more about concentration here
https://brainly.com/question/14312118
#SPJ4
The normal boiling point of ethanol is 78.8°c. the heat of vaporization of ethanol is 43.5 kj/mol. what is δs for the vaporization of 1 mole of ethanol at 78.8°c?
Answers
To find the entropy change (δs) for the vaporization of 1 mole of ethanol at its normal boiling point of 78.8°C, we can use the Clausius-Clapeyron equation:
ln(P2/P1) = (-ΔHvap/R) x (1/T2 - 1/T1)
where P1 and P2 are the initial and final pressures, ΔHvap is the heat of vaporization, R is the gas constant, and T1 and T2 are the initial and final temperatures.
At the normal boiling point of ethanol, the initial pressure is atmospheric pressure (1 atm) and the final pressure is also 1 atm (since the ethanol is boiling at its normal boiling point). Therefore, ln(P2/P1) = 0.
Substituting the given values, we get:
0 = (-43.5 kJ/mol / 8.314 J/molK) x (1/351.95 K - 1/351.95 K)
Solving for δs, we get:
δs = ΔSvap = -ΔHvap / T
where T is the temperature in Kelvin. Plugging in the values, we get:
δs = (-43.5 kJ/mol) / (351.95 K) = -0.124 kJ/molK
Therefore, the entropy change for the vaporization of 1 mole of ethanol at its normal boiling point of 78.8°C is -0.124 kJ/molK.
To find the change in entropy (δS) for the vaporization of 1 mole of ethanol at 78.8°C, we'll use the formula:
δS = (Heat of Vaporization) / (Temperature in Kelvin)
To know more about Vaporization visit:
https://brainly.com/question/12625048
#SPJ11
a cr3 (aq)cr3 (aq) solution is electrolyzed using a current of 6.00 aa. part a what mass of cr(s)cr(s) is plated out after 2.20 days? What amperage is required to plate out 0.250mol Cr from a Cr3+ solution in a period of 8.60h ?
Answers
1. 0.134 g of Cr is plated out after 2.20 days.
2. 1.39 A of current is required to plate out 0.250 mol Cr in 8.60 h.
To calculate the mass of Cr plated out, we need to use Faraday's law of electrolysis, which states that the amount of substance plated out is directly proportional to the quantity of electricity passed through the solution.
The formula is:
moles of substance plated out = (current x time) / (96500 x number of electrons transferred)
For Cr, the number of electrons transferred is 3, so the formula becomes:
moles of Cr plated out = (6.00 A x 2.20 days x 24 h/day x 3600 s/h) / (96500 x 3)
Solving for moles, we get 0.250 mol. To convert to mass, we use the molar mass of Cr, which is 52.00 g/mol. Therefore, the mass of Cr plated out is:
mass of Cr = 0.250 mol x 52.00 g/mol = 13.0 g = 0.134 g
For the second part of the question, we need to rearrange the formula to solve for the current:
current = (moles of substance plated out x 96500 x number of electrons transferred) / (time)
Plugging in the values, we get:
current = (0.250 mol x 96500 x 3) / (8.60 h x 3600 s/h) = 1.39 A.
For more such questions on current, click on:
https://brainly.com/question/1100341
#SPJ11
When a Cr⁺³ solution is electrolyzed, the ions undergo a reduction reaction to form solid chromium on the cathode. The balanced equation for this reaction is:
2Cr⁺³ + 6e- → 2Cr(s)
To calculate the mass of chromium plated out after 2.20 days, we need to first determine the amount of charge (Q) that has passed through the cell:
Q = I × t
where I is the current in amperes and t is the time in seconds.
Converting 2.20 days to seconds:
2.20 days × 24 hours/day × 60 minutes/hour × 60 seconds/minute = 190,080 seconds
So, Q = 6.00 A × 190,080 s = 1.14 × 10⁺⁶ C
Next, we can use Faraday's law to calculate the amount of chromium plated out:
moles of e- = Q / F
where F is the Faraday constant (96,485 C/mol e-), so
moles of e- = 1.14 × 10⁺⁶ C / 96,485 C/mol e- = 11.8 mol e-
Since each mole of electrons reduces 2 moles of Cr⁺³ to form 1 mole of Cr, we have:
moles of Cr = 11.8 mol e- × 1 mol Cr⁺³ / 2 mol e- = 5.90 mol Cr
Finally, we can use the molar mass of chromium (52.0 g/mol) to calculate the mass of chromium plated out:
mass of Cr = 5.90 mol Cr × 52.0 g/mol = 307 g Cr
Therefore, after 2.20 days of electrolysis with a current of 6.00 A, 307 g of chromium is plated out.
To determine the amperage required to plate out 0.250 mol of Cr from a Cr⁺³ solution in 8.60 hours, we can use a similar approach.
First, we need to convert the time to seconds:
8.60 hours × 60 minutes/hour × 60 seconds/minute = 30,960 seconds
Next, we can use the same equation as before to calculate the amount of charge required:
Q = (0.250 mol × 3 mol e- / 2 mol Cr⁺³) × (96,485 C/mol e-) = 36,368 C
Finally, we can use the equation for current (I = Q / t) to find the required amperage:
I = 36,368 C / 30,960 s = 1.17 A
Therefore, a current of 1.17 A is required to plate out 0.250 mol of chromium from a Cr⁺³ solution in 8.60 hours.
Learn more about electrolysis here:
https://brainly.com/question/12054569
#SPJ11
Place the following in order of decreasing magnitude of lattice energy NaF RbBr KCI A) RbBr > NaF > KCI B) NaF> KCI> RbBr C) KCI NaF > RbBr D) NaF> RbBr > KCI O D O A O B O C
Answers
D
O A
O B O C
NaF > RbBr > KCI
something like that
NaF has the smallest ion size and highest charge (Na+ and F-), leading to the highest lattice energy. RbBr has a larger ion size and lower charge (Rb+ and Br-), resulting in lower lattice energy than NaF but still higher than KCI.
D) NaF> RbBr > KCI
The order of decreasing magnitude of lattice energy is determined by the ionic size and charge of the ions. Smaller ions with higher charges will have stronger attraction between them, resulting in higher lattice energy.
NaF has the smallest ion size and highest charge (Na+ and F-), leading to the highest lattice energy. RbBr has a larger ion size and lower charge (Rb+ and Br-), resulting in lower lattice energy than NaF but still higher than KCI. KCI has the largest ion size and lowest charge (K+ and Cl-), giving it the lowest lattice energy of the three compounds.
For more information on lattice energy visit:
brainly.com/question/31730061
#SPJ11
A single phase 2500/250V, two winding ideal transformer has a load of 10 < 40°Ω connected t its secondary. If the primary of the transformer is connected to a 2400 V line, determine the following: a. The secondary current b. The primary current c. The input impedance as seen from the line d. The output power of the transformer in kVA and in kw e. The input power of the transformer in kVA and in kw
Answers
Answer is a. The secondary current: 25 < -40°A, b. The primary current: 2.604 < -40°A, c. input impedance as seen from the line: 96 < 40°Ω, d. output power in kVA and kW: 4.79kW, e. input power in kVA and kW: 4.79kW.
a. The secondary current: To find the secondary current, divide the secondary voltage by the impedance of the load: Is = Vs / Z_load. Is = 250V / (10 < 40°Ω) = 25 < -40°A.
b. The primary current: The transformer ratio is N1/N2 = 2400/250 = 9.6. The primary current is then Ip = Is / (N1/N2) = 25 < -40°A / 9.6 = 2.604 < -40°A.
c. The input impedance as seen from the line: Z_input = N1/N2 * Z_load = 9.6 * (10 < 40°Ω) = 96 < 40°Ω.
d. The output power in kVA and kW: The apparent power (S) is calculated as S = Vs * Is = 250V * 25 < -40°A = 6.25kVA. The real power (P) is P = S * power factor = 6.25kVA * cos(40°) = 4.79kW.
e. The input power in kVA and kW: For an ideal transformer, the input and output power are equal. Therefore, the input power in kVA is 6.25kVA, and the input power in kW is 4.79kW.
To know more about current visit:
brainly.com/question/16255130
#SPJ11
how many hydrogens are in c9h?no, which has 1 ring(s) and 3 double bond(s)?
Answers
12 hydrogens in the molecule[tex]C_9H_1_2NO[/tex] with 1 ring and 3 double bonds.
To determine how many hydrogens are in the molecule C9H?NO with 1 ring and 3 double bonds, follow these steps:
1. Calculate the number of hydrogen atoms required for a fully saturated molecule using the formula H = 2C + 2, where C is the number of carbon atoms. In this case, C = 9.
H = 2(9) + 2 = 18 + 2 = 20
2. Subtract the hydrogen atoms corresponding to the presence of the ring and double bonds. Each double bond and ring removes 2 hydrogen atoms from the fully saturated molecule.
Total removed hydrogens = 2(double bonds) + 2(rings) = 2(3) + 2(1) = 6 + 2 = 8
3. Calculate the actual number of hydrogen atoms in the molecule by subtracting the removed hydrogens from the fully saturated molecule.
Actual hydrogens = H - Total removed hydrogens = 20 - 8 = 12
So, there are 12 hydrogens in the molecule[tex]C_9H_1_2NO[/tex] with 1 ring and 3 double bonds.
To know more about Hydrogen refer here :
https://brainly.com/question/24433860
#SPJ11
The following compounds are treated with HNO3/H2SO4. Predict the positions of electrophilic attack that lead to the major nitration product(s).
(Enter each possible nitration position as an alphabetical letter string without commas or spaces, i.e. ab or abd. Enter the string in alphabetical order.)
Answers
When a compound is treated with HNO3/H2SO4, it undergoes nitration, which is a type of electrophilic aromatic substitution. Nitration involves the substitution of a nitro group (-NO2) onto an aromatic ring.
The positions of electrophilic attack that lead to the major nitration product(s) depend on the structure of the compound. In general, electron-rich aromatic rings are more susceptible to nitration because they are better able to stabilize the intermediate cationic species that is formed during the reaction.
To predict the positions of electrophilic attack, we need to identify the electron-rich positions on the aromatic ring. The most electron-rich positions are ortho and para to any electron-donating substituents on the ring, such as alkyl groups (-CH3) or hydroxy groups (-OH). The meta position is less electron-rich because it is further away from the electron-donating substituent.
To know more about undergoes nitration visit:-
https://brainly.com/question/7008207
#SPJ11
What is the value of ΔG at 120. 0 K for a reaction in which ΔH = +35 kJ/mol and ΔS = -1. 50 kJ/(mol·K)?
Answers
The value of ΔG at 120.0 K for the given reaction is +215 kJ/mol.To calculate the value of ΔG (change in Gibbs free energy) at 120.0 K for a reaction, we can use the equation: ΔG = ΔH - TΔS
Where:
ΔG is the change in Gibbs free energy (in kJ/mol)
ΔH is the change in enthalpy (in kJ/mol)
T is the temperature (in Kelvin)
ΔS is the change in entropy (in kJ/(mol·K))
Given:
ΔH = +35 kJ/mol
ΔS = -1.50 kJ/(mol·K)
T = 120.0 K
Substituting the given values into the equation, we have:
ΔG = +35 kJ/mol - (120.0 K)(-1.50 kJ/(mol·K))
ΔG = +35 kJ/mol + 180 kJ/mol
ΔG = 215 kJ/mol
Therefore, the value of ΔG at 120.0 K for the given reaction is +215 kJ/mol.
To learn more about reaction click here:
brainly.com/question/31425638
#SPJ11
make a table on hazadous gases and its effects
Answers
Hazardous gases are those that can cause harm to humans, animals, and the environment. Some of the most dangerous gases include carbon monoxide, sulfur dioxide, nitrogen oxides, and volatile organic compounds.
Gas Name of Gas Effects on Health Effects on the Environment Carbon Monoxide Colorless, odorless gas It can cause headaches, dizziness, nausea, vomiting, and even death in severe cases.
Air pollution, climate change Sulfur Dioxide A colorless gas with a pungent odor Irritation of the eyes, nose, throat, and respiratory system.
It can lead to bronchoconstriction, reduced lung function, and increased risk of respiratory infection.
Acid rain, soil and water pollution Nitrogen Oxides A group of gases including nitrogen monoxide, nitrogen dioxide, and nitrous oxide Respiratory problems, such as coughing, wheezing, and shortness of breath. It can also increase the risk of respiratory infections.
Acid rain, smog, ground-level ozone formationVolatile Organic Compounds (VOCs)A group of chemicals that includes benzene, formaldehyde, and toluene.
Headaches, nausea, and other health effects, including cancer. VOCs contribute to the formation of ozone in the lower atmosphere (troposphere), which can lead to respiratory problems and other health effects.
In conclusion, hazardous gases can have serious effects on human health and the environment.
It is important to take steps to reduce the emission of these gases, such as using clean energy sources, reducing the use of fossil fuels, and adopting environmentally friendly practices.
For more questions on Hazardous gases
https://brainly.com/question/29830849
#SPJ8
3. For the following balanced redox reaction answer the following questions 4NaOH(aq)+Ca(OH) 2
(aq)+C(s)+4ClO 2
( g)→4NaClO 2
(aq)+CaCO 3
( s)+3H 2
O(l) a. What is the oxidation state of Cl in ClO 2
( g) ? b. What is the oxidation state of C in C(s) ? c. What is the element that is oxidized? d. What is the element that is reduced? e. What is the oxidizing agent? f. What is the reducing agent? g. How many electrons are transferred in the reaction as it is balanced?
Answers
a. The oxidation state of Cl in ClO₂(g) is +3.
b. The oxidation state of C in C(s) is 0.
c. The element that is oxidized is Cl.
d. The element that is reduced is C.
e. The oxidizing agent is ClO₂.
f. The reducing agent is C.
g. To balance the equation, 3 electrons are transferred in each of the 4 half-reactions. Therefore, a total of 12 electrons are transferred in the reaction.
Oxidation and reduction are chemical processes that involve the transfer of electrons between reactant species. Oxidation refers to the loss of electrons by a reactant species, resulting in an increase in its oxidation state. Reduction, on the other hand, refers to the gain of electrons by a reactant species, resulting in a decrease in its oxidation state.
An easy way to remember these processes is through the mnemonic "OIL RIG", which stands for "Oxidation Is Loss, Reduction Is Gain". In an oxidation-reduction (redox) reaction, one species undergoes oxidation while another undergoes reduction.
Learn more about the oxidation and reduction: https://brainly.com/question/13699873
#SPJ11
If 10.0 grams of AI(OH) react with 10.0 grams of H2S04...
a.
Determine the limiting reactant and the excess reactant.
b.
Using your information from part a, predict the mass, in grams, of H2O vou expect to produce.
Answers
Taking into account the reaction stoichiometry, H₂SO₄ will be the limiting reagent and 3.67 grams of H₂O are formed if 10.0 grams of AI(OH)₃ react with 10.0 grams of H₂SO₄.
Reaction stoichiometryIn first place, the balanced reaction is:
2 Al(OH)₃ + 3 H₂SO₄ → Al₂(SO₄)₃ + 6 H₂O
By reaction stoichiometry (that is, the relationship between the amount of reagents and products in a chemical reaction), the following amounts of moles of each compound participate in the reaction:
Al(OH)₃: 2 molesH₂SO₄: 3 molesAl₂(SO₄)₃: 1 moleH₂O: 6 molesThe molar mass of the compounds is:
Al(OH)₃: 78 g/moleH₂SO₄: 98 g/moleAl₂(SO₄)₃: 342 g/moleH₂O: 18 g/moleBy reaction stoichiometry, the following mass quantities of each compound participate in the reaction:
Al(OH)₃: 2 moles ×78 g/mole= 156 gramsH₂SO₄: 3 moles ×98 g/mole= 294 gramsAl₂(SO₄)₃: 1 mole ×342 g/mole= 342 gramsH₂O: 6 moles ×18 g/mole= 108 gramsLimiting reagentThe limiting reagent is one that is consumed first in its entirety, determining the amount of product in the reaction. When the limiting reagent is finished, the chemical reaction will stop.
To determine the limiting reagent, it is possible to use a simple rule of three as follows: if by stoichiometry 156 grams of Al(OH)₃ reacts with 294 grams of H₂SO₄, 10 grams of Al(OH)₃ reacts with how much mass of H₂SO₄?
mass of H₂SO₄= (10 grams of Al(OH)₃×294 grams of H₂SO₄)÷156 grams of Al(OH)₃
mass of H₂SO₄= 18.85 grams
But 18.85 grams of H₂SO₄ are not available, 10 grams are available. Since you have less mass than you need to react with 10 grams of Al(OH)₃, H₂SO₄ will be the limiting reagent.
Mass of H₂O formedThe following rule of three can be applied: if by reaction stoichiometry 294 grams of H₂SO₄ form 108 grams of H₂O, 10 grams of H₂SO₄ form how much mass of H₂O?
mass of H₂O= (10 grams of H₂SO₄×108 grams of H₂O)÷294 grams of H₂SO₄
mass of H₂O= 3.67 grams
Finally, 3.67 grams of H₂O are formed.
Learn more about the reaction stoichiometry:
brainly.com/question/24741074
#SPJ1
in the production of potassium metal, the source of electrons in the reduction of k ions is a. h2(g). b. co(g). c. cao(s). d. electrolysis.
Answers
The production of potassium metal involves the reduction of potassium ions (K+) to form elemental potassium (K). This reduction process requires a source of electrons. the correct answer is (d) electrolysis.
In the case of potassium metal production, electrolysis is used to provide the necessary electrons.
During the electrolysis process, an external electric field is applied to an electrolytic cell containing a potassium-containing solution, causing K+ ions to be attracted to the negatively charged electrode (cathode) and gain electrons.
As a result, the K+ ions are reduced to form potassium atoms (K), which are deposited on the cathode surface to form metallic potassium. Therefore, the correct answer is (d) electrolysis.
To know more about electrolysis, refer here:
https://brainly.com/question/12054569#
#SPJ11
A solution is prepared in which a small amount of Fe^2+ is added to a much larger amount of solution in which?
the [OH-] is 1.0 x 10^-2M. Some Fe(OH)2 precipitates. The value of Ksp for Fe(OH)2 = 8.0 x 10^-10.
a.) Assuming that the hydrozide concentration is 1.0 x 10^-2M, calculate the concentration of Fe2+ in solution
b.) A battery is prepared using the above solution with an iron wire dipping into it as one half-cell. The other half-cell is the standard nickel electrode. Write the balanced net ionic equation for the cell reaction
c.) use the nernst equation to calculate the potential of the above cell.
Answers
A. The concentration of Fe^2+ in solution is 8.0 × 10^-6 M.
B. The balanced net ionic equation for the cell reaction is:
Fe(s) + Ni^2+(aq) → Fe^2+(aq) + Ni(s)
C. The potential of the cell is 0.34 V.
a) The balanced chemical equation for the precipitation reaction is:
Fe^2+(aq) + 2OH^-(aq) → Fe(OH)2(s)
The solubility product expression for Fe(OH)2 is
Ksp = [Fe^2+][OH^-]^2
At equilibrium, the concentrations of Fe^2+ and OH^- are related to Ksp as follows:
Ksp = [Fe^2+][OH^-]^2
Rearranging this equation gives:
[Fe^2+] = Ksp/[OH^-]^2
Substituting the given values gives:
[Fe^2+] = (8.0 × 10^-10)/(1.0 × 10^-2)^2 = 8.0 × 10^-6 M
b) The balanced net ionic equation for the cell reaction is:
Fe(s) + Ni^2+(aq) → Fe^2+(aq) + Ni(s)
c) The Nernst equation relates the cell potential (Ecell) to the standard cell potential (E°cell), the reaction quotient (Q), and the temperature (T):
Ecell = E°cell - (RT/nF) ln(Q)
where R is the gas constant (8.314 J/(mol K)), T is the temperature in kelvin, F is the Faraday constant (96,485 C/mol), n is the number of electrons transferred in the reaction (2 in this case), and ln is the natural logarithm.
At standard conditions (1 M concentration and 25°C temperature), the standard cell potential for the Fe/Ni half-cell reaction is:
E°cell = E°cathode - E°anode = 0.00 V - (-0.44 V) = 0.44 V
To calculate the cell potential at non-standard conditions, we need to calculate the reaction quotient Q. The concentrations of Fe^2+ and Ni^2+ are given, but we need to calculate the concentration of OH^- in the Fe/Ni half-cell. At the cathode (Fe electrode), the following reaction occurs:
Fe^2+(aq) + 2e^- → Fe(s)
The Fe electrode will consume Fe^2+ ions in solution, causing the OH^- concentration to increase. We can assume that the Fe(OH)2 precipitate formed in part a) is negligibly small compared to the OH^- concentration in solution.
Since the overall reaction involves the transfer of 2 electrons, we need to balance the half-cell reactions so that the number of electrons transferred is the same:
Fe(s) → Fe^2+(aq) + 2e^- (oxidation)
Ni^2+(aq) + 2e^- → Ni(s) (reduction)
The standard reduction potential for the Ni^2+/Ni half-cell is -0.44 V. Using the Nernst equation, the cell potential at non-standard conditions is:
Ecell = E°cell - (RT/nF) ln(Q)
Q = [Fe^2+]/[Ni^2+]
[OH^-] = (Ksp/[Fe^2+])^(1/2)
Now substituting the values of Q and E°cell in the Nernst equation gives:
Ecell = 0.44 V - (8.314 J/(mol K) × 298 K)/(2 × 96,485 C/mol) × ln(8.0) = 0.34 V
For more question on concentration click on
https://brainly.com/question/26255204
#SPJ11
hydrogen bonding between the carbonyl group of an amino acid on one strand with the amino group of the neighboring strand leads to ______.
Answers
Hydrogen bonding between the carbonyl group of one amino acid on a polypeptide chain with the amino group of another amino acid on the neighboring chain leads to the formation of alpha helix or beta pleated sheet in proteins.
This type of bonding occurs due to the electronegativity difference between nitrogen and oxygen atoms, which leads to a partial negative charge on the oxygen atom and a partial positive charge on the hydrogen atom of the amino group.
This partial charge allows the oxygen to form a hydrogen bond with the hydrogen of the carbonyl group on the neighboring strand, resulting in the formation of a stable protein structure.
To know more about Hydrogen bonding, refer here:
https://brainly.com/question/15099999#
#SPJ11
14. what would be the effect of a mutation in an allosteric enzyme that resulted in a t/r ratio of 0? (
Answers
A mutation in an allosteric enzyme that causes a t/r ratio of 0 would likely result in the complete loss of regulatory function, leading to a constitutively active enzyme that is insensitive to allosteric modulation.
Allosteric enzymes are proteins that can change their conformation and activity in response to the binding of specific molecules at sites other than the active site. The t/r ratio, also known as the relaxed/tense ratio, refers to the equilibrium between the active (relaxed) and inactive (tense) states of the enzyme. A t/r ratio of 0 implies that the enzyme exists solely in the tense state, with no active conformation.
When an allosteric enzyme is in a tense state, it typically exhibits reduced or no activity. The relaxed state, on the other hand, corresponds to the active form of the enzyme.
In normal conditions, allosteric regulation allows the enzyme to switch between these two states, controlling its activity based on the presence or absence of specific molecules. However, a mutation that leads to a t/r ratio of 0 indicates that the enzyme is locked in the inactive tense state and cannot transition to the active relaxed state.
Consequently, the enzyme loses its ability to respond to allosteric modulators, resulting in a constitutively active enzyme that operates independently of regulatory signals. This can have significant implications for cellular processes, potentially leading to dysregulation of metabolic pathways and disrupted physiological functions.
Learn more about allosteric enzyme here:
https://brainly.com/question/27961730
#SPJ11
many oxide ceramics or ionic compounds have moduli of elasticity around 6.9x104 mpa, independent of composition. why
Answers
Many oxide ceramics and ionic compounds exhibit similar moduli of elasticity, typically around 6.9x104 MPa, regardless of their chemical composition or structure.
Oxide ceramics and ionic compounds are known for their high strength and excellent mechanical properties, making them useful for a variety of applications in industries such as aerospace, energy, and electronics. One of the key properties that govern their mechanical behavior is the modulus of elasticity, which measures the material's resistance to deformation under applied stress.
This phenomenon can be explained by considering the bonding nature of these materials. Oxide ceramics and ionic compounds are characterized by strong ionic bonds between positively and negatively charged ions, which result in a highly ordered crystal lattice structure. Because the bonding interactions are primarily electrostatic in nature, they do not depend strongly on the specific chemical composition of the material, but rather on the arrangement of the ions within the lattice. As a result, the modulus of elasticity is largely independent of the material's composition.
Of course, there are some exceptions to this general trend, as certain factors such as crystal defects, grain boundaries, and impurities can influence the mechanical properties of oxide ceramics and ionic compounds. Nonetheless, the relatively consistent modulus of elasticity observed across a wide range of materials in this class highlights the importance of understanding the fundamental bonding principles that govern their behavior.
To know more about chemical composition or structure visit:
https://brainly.com/question/16997829
#SPJ11